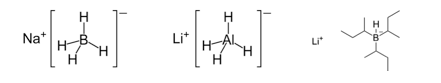
The control of remote asymmetric centres via reduction of acyclic carbonyl functions - Journal of the Chemical Society, Perkin Transactions 1 (RSC Publishing)
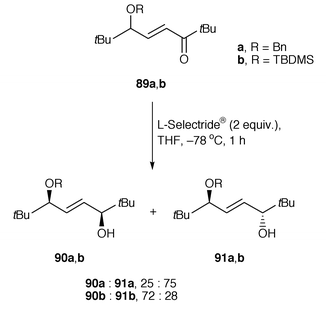
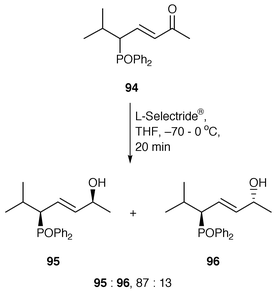
The control of remote asymmetric centres via reduction of acyclic carbonyl functions - Journal of the Chemical Society, Perkin Transactions 1 (RSC Publishing)
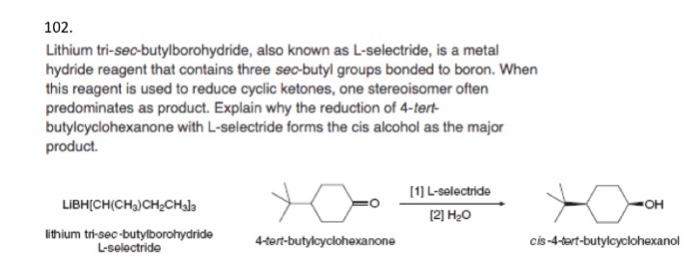
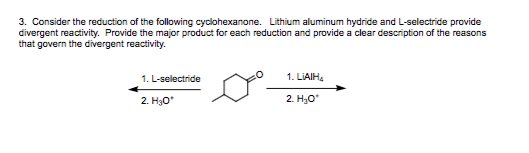
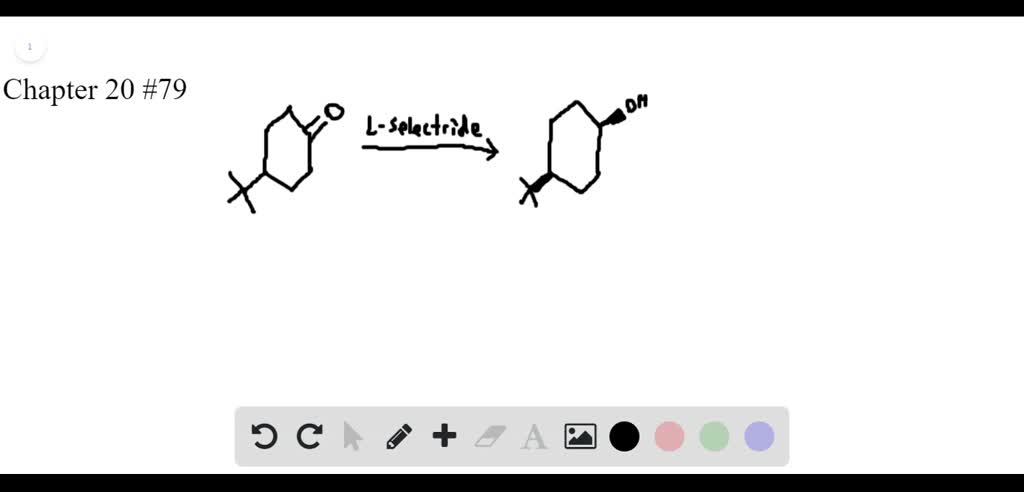
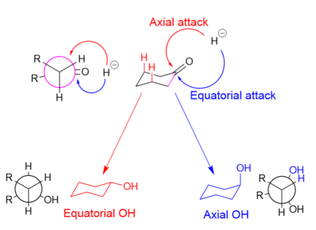
Course Name: Methods in Organic Synthesis Paper Number: 202 Section: B (Group V and VI) Course Instructor: Dr Trapti Aggarwal

NABH4, NaBH4/CeCl3, NaBH3CN, L-Selectride & K-Selectride Reducing Regents Reaction & Mechanism - YouTube

Stereoselective syntheses of galanthamine and its stereoisomers by complementary Luche and L-selectride reductions - ScienceDirect
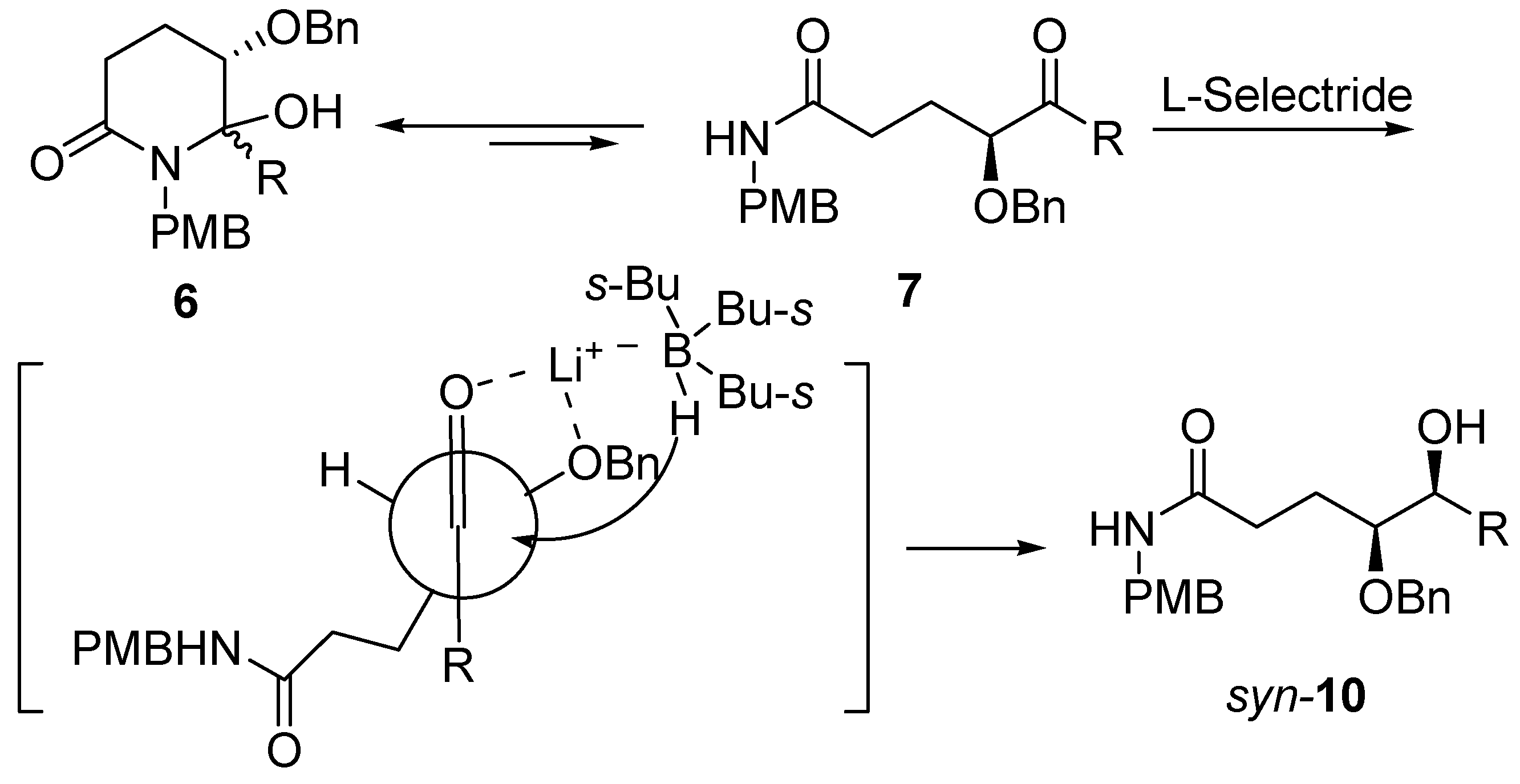
Molecules | Free Full-Text | A General and Simple Diastereoselective Reduction by L-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides | HTML
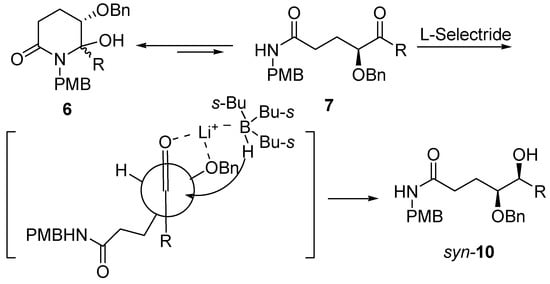
Molecules | Free Full-Text | A General and Simple Diastereoselective Reduction by L-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides | HTML

Aspects of stereocontrol in the L-Selectride reduction of 4-acyl-1,3-dioxolane derivatives - ScienceDirect

Aspects of stereocontrol in the L-Selectride reduction of 4-acyl-1,3-dioxolane derivatives - ScienceDirect

Molecules | Free Full-Text | A General and Simple Diastereoselective Reduction by L-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides | HTML