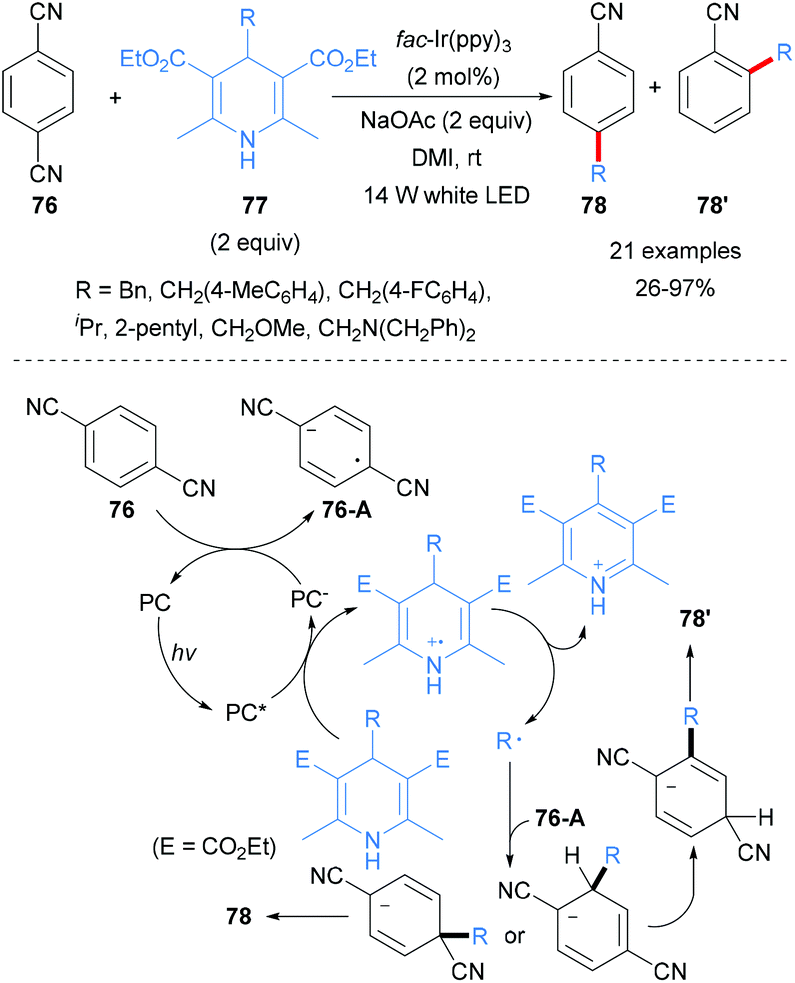
Hantzsch Ester as a Visible‐Light Photoredox Catalyst for Transition‐Metal‐Free Coupling of Arylhalides and Arylsulfinates - Zhu - 2020 - Chemistry – A European Journal - Wiley Online Library

Mechanism of the highly selective transfer hydrogenation using Hantzsch... | Download Scientific Diagram
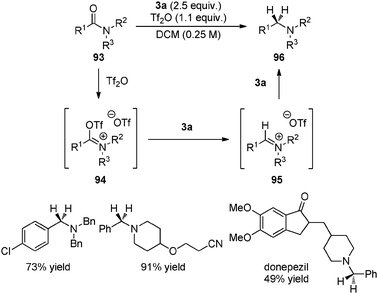
Transfer hydrogenation with Hantzsch esters and related organic hydride donors - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C1CS15268H
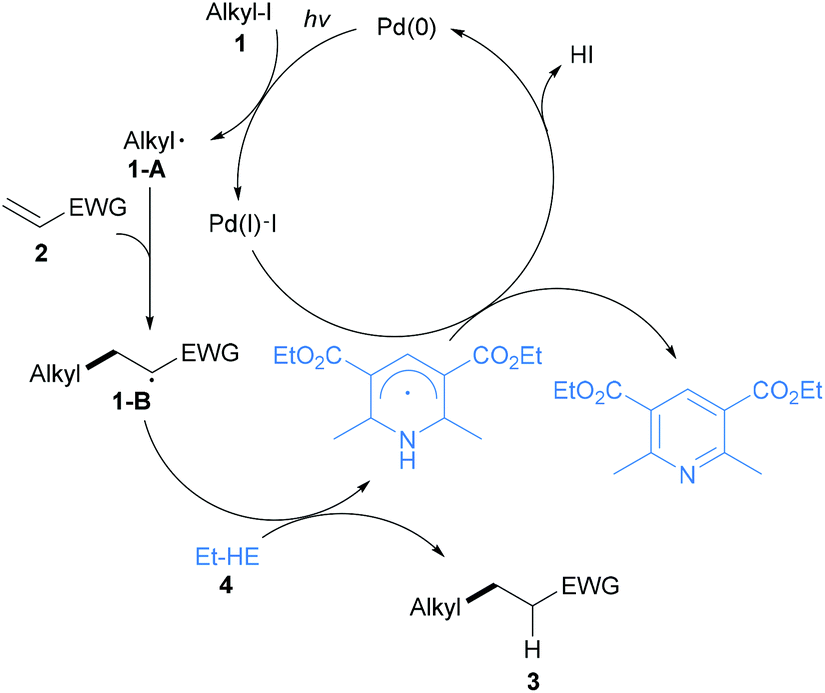
Hantzsch esters: an emerging versatile class of reagents in photoredox catalyzed organic synthesis - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C9OB01289C
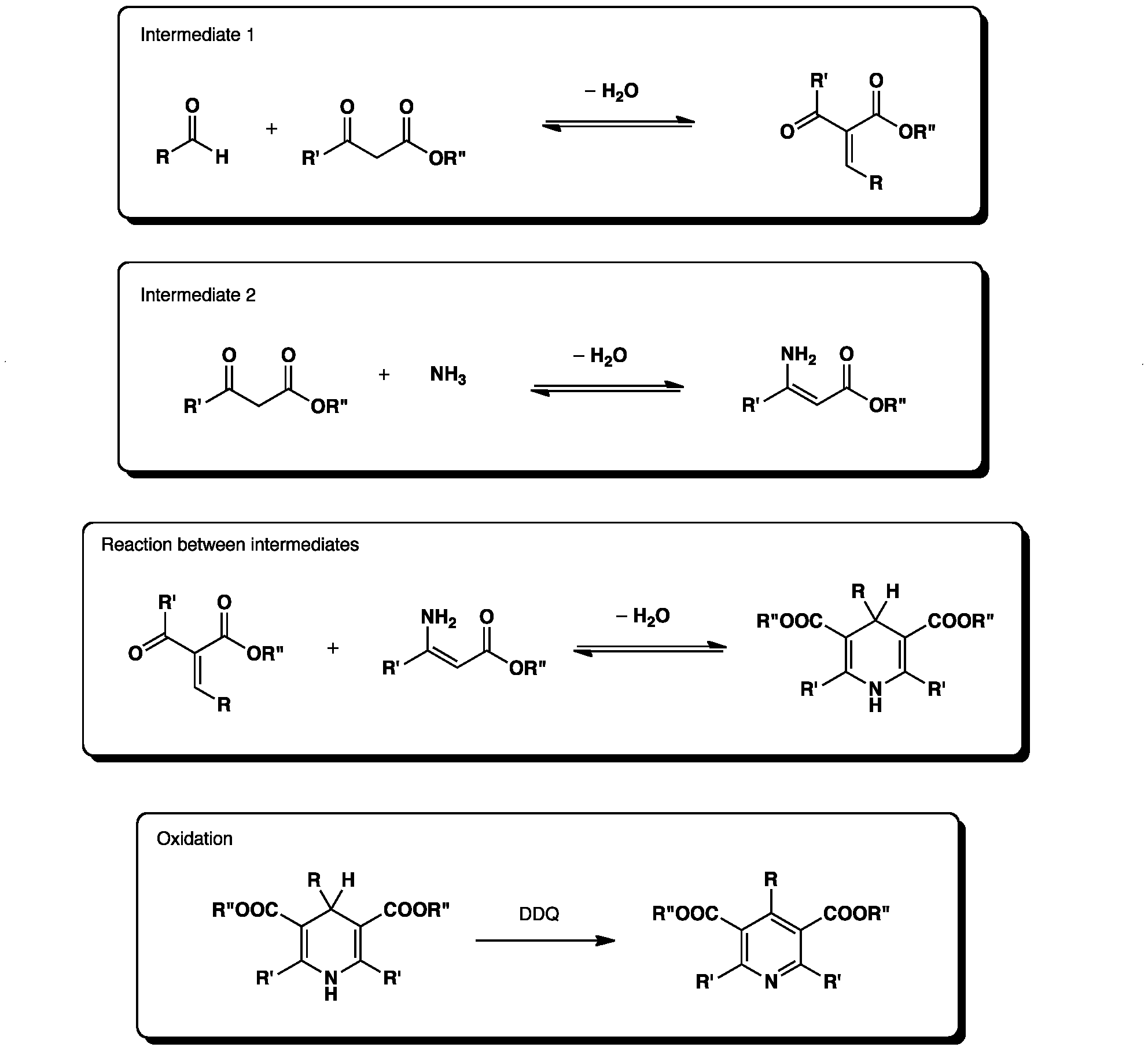
![Fesipmim]Cl as highly efficient and reusable catalyst for solventless synthesis of dihydropyridine derivatives through Hantzsch reaction | SpringerLink Fesipmim]Cl as highly efficient and reusable catalyst for solventless synthesis of dihydropyridine derivatives through Hantzsch reaction | SpringerLink](https://media.springernature.com/lw685/springer-static/image/art%3A10.1007%2Fs12039-020-01770-9/MediaObjects/12039_2020_1770_Figa_HTML.png)
Fesipmim]Cl as highly efficient and reusable catalyst for solventless synthesis of dihydropyridine derivatives through Hantzsch reaction | SpringerLink
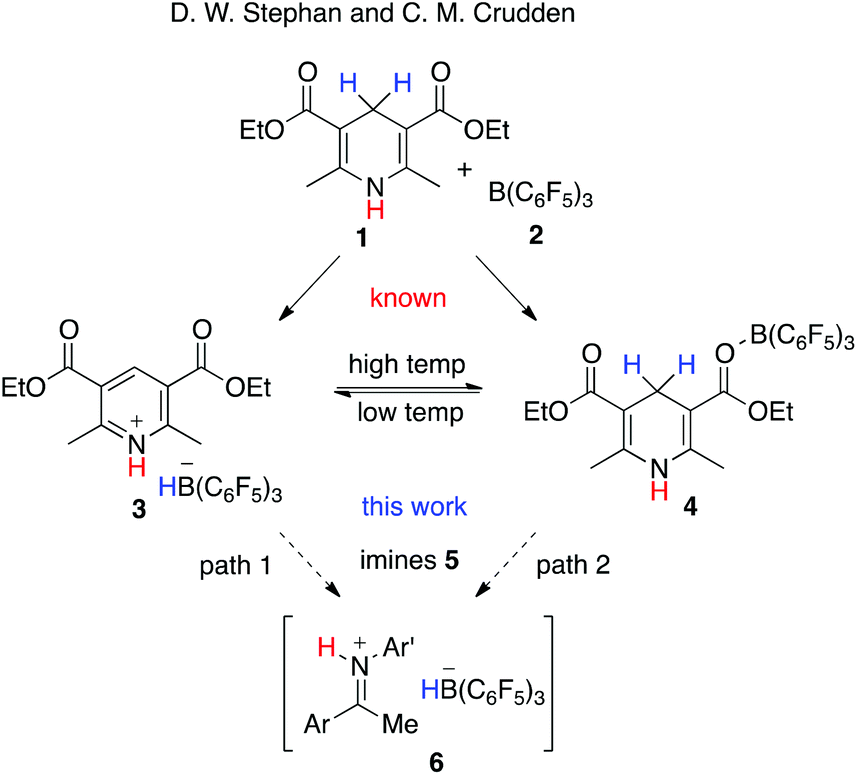
B(C 6 F 5 ) 3 -catalyzed transfer hydrogenations of imines with Hantzsch esters - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB00023A

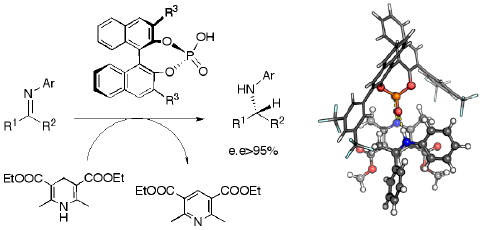
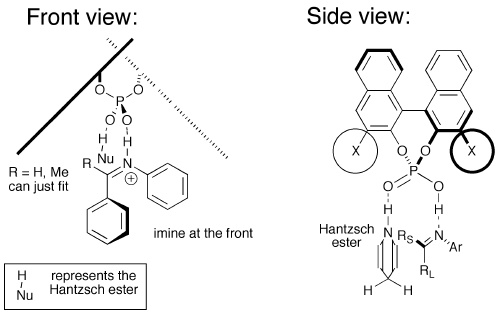
Recent Developments in Asymmetric Transfer Hydrogenation with Hantzsch Esters: A Biomimetic Approach - You - 2007 - Chemistry – An Asian Journal - Wiley Online Library
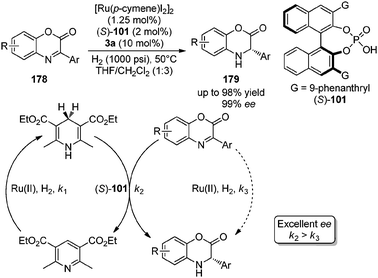
Transfer hydrogenation with Hantzsch esters and related organic hydride donors - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C1CS15268H

Hantzsch Ester as a Visible‐Light Photoredox Catalyst for Transition‐Metal‐Free Coupling of Arylhalides and Arylsulfinates - Zhu - 2020 - Chemistry – A European Journal - Wiley Online Library

On-water” reduction of α-keto amide by Hantzsch ester: A chemoselective catalyst- and additive-free way to α-hydroxy amide - ScienceDirect